Chelate, the chelate formed by chelating agents, comes from the Greek word Chele, meaning crab claw. Chelates are like crab claws holding metal ions, which are highly stable and easy to remove or use these metal ions. In 1930, the first chelate was synthesized in Germany - EDTA (ethylenediamine tetraacetic acid) chelate for the treatment of heavy metal poisoning patients, and then the chelate was developed and applied to daily chemical washing, food, industry and other applications.
At present, the main manufacturers of chelating agents in the world include BASF, Norion, Dow, Dongxiao Biological, Shijiazhuang Jack and so on.
The Asia-Pacific region is the largest market for chelating agents, with more than 50% share and an estimated market size of more than US $1 billion, with mainstream applications in the detergent, water treatment, personal care, paper, food and beverage industries.
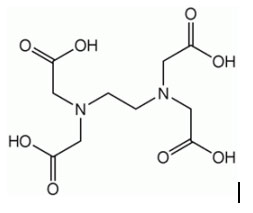
(Molecular structure of the chelating agent EDTA)
Chelating agents control metal ions by chelating their multi-ligands with metal ion complexes to form chelates.
From this mechanism, it can be understood that many molecules with multi-ligands have such chelation ability.
One of the most typical is the above EDTA, which can provide 2 nitrogen atoms and 4 carboxyl oxygen atoms to cooperate with the metal, and can use 1 molecule to tightly wrap the calcium ion that needs 6 coordination, generating a very stable product with excellent chelation ability. Other commonly used chelators include sodium phytate such as sodium gluconate, sodium glutamate diacetate tetrasodium (GLDA), sodium amino acids such as methylglycine diacetate trisodium (MGDA), and polyphosphates and polyamines.
As we all know, whether in tap water or in natural water bodies, there are calcium, magnesium, iron plasma, these metal ions in the long-term enrichment, will bring the following effects on our daily life:
1. The fabric is not properly cleaned, causing scale deposition, hardening and darkening.
2. There is no suitable cleaning agent on the hard surface, and scale deposits
3. Scale deposits in tableware and glassware
Water hardness refers to the content of calcium and magnesium ions in water, and hard water will reduce the washing effect. In detergent products, the chelating agent can react with calcium, magnesium and other metal ions in the water, so as to soften the water quality, prevent calcium and magnesium plasma from reacting with the active agent in the detergent, and avoid affecting the washing effect, thereby improving the effectiveness of the washing product.
In addition, chelating agents can also make the composition of the detergent more stable and less susceptible to decomposition when heated or stored for a long time.
The addition of chelating agent to laundry detergent can enhance its cleaning power, especially in areas where the washing effect is greatly affected by hardness, such as the north, southwest and other areas with high water hardness, chelating agent can also prevent water stains and stains from settling on the surface of the fabric, so that the laundry detergent is more permeable and more easily adhered to the surface of the clothing, improving the washing effect at the same time. Improve whiteness and softness, intuitive performance is not so gray and dry hard.
Also in hard surface cleaning and tableware cleaning, the chelating agent in the detergent can improve the dissolution and dispersion ability of the detergent, so that the stain and scale are easier to remove, and the intuitive performance is that the scale can not remain, the surface is more transparent, and the glass does not hang water film. Chelating agents can also combine with oxygen in the air to form stable complexes that inhibit the oxidation of metal surfaces.
In addition, the chelating effect of chelating agents on iron ions is also used in pipe cleaners for rust removal.
Post time: Jul-03-2024







